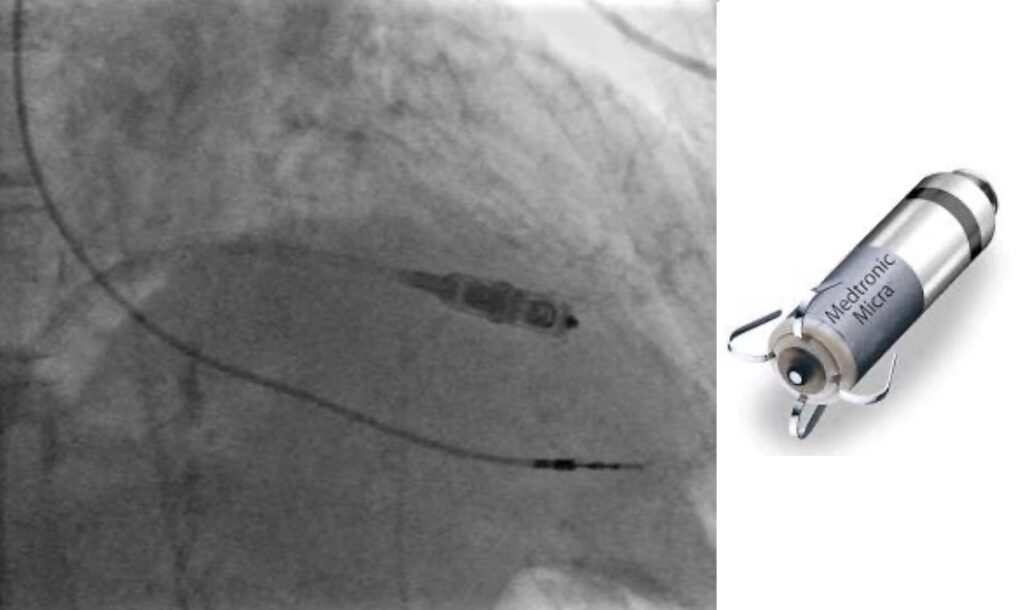
Figure 1. Leadless pacemaker insertion and device (Medtronic Micra)
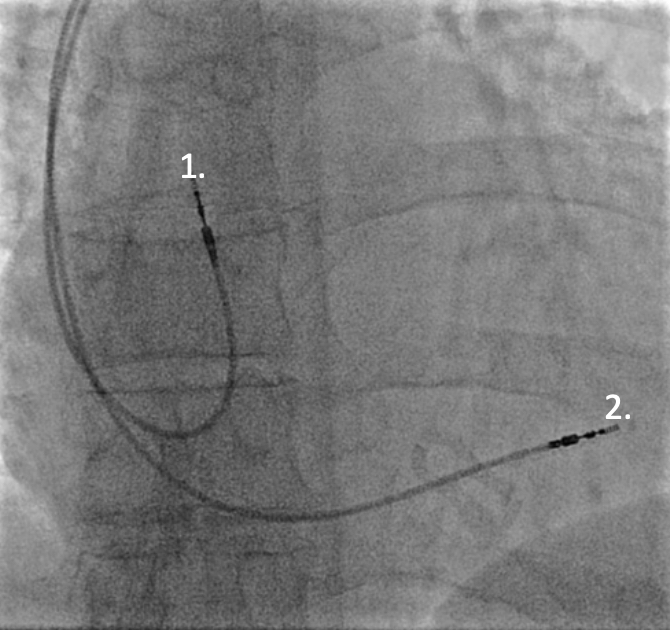
Dual Chamber Pacing
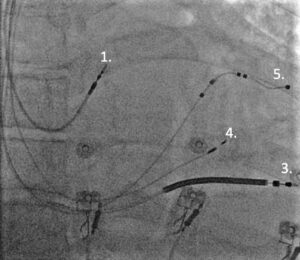
LOT-CRT Pacing
Figure 2: Conventional dual chamber pacing (top) and Cardiac resynchronisation therapy utilising a conduction system pacing technique (left bundle branch pacing) combined with conventional left ventricular lead implantation.
1. High right atrial lead
2. Right ventricular lead
3. Right ventricular defibrillator lead
4. Left bundle branch pacing lead
5. Coronary sinus pacing lead
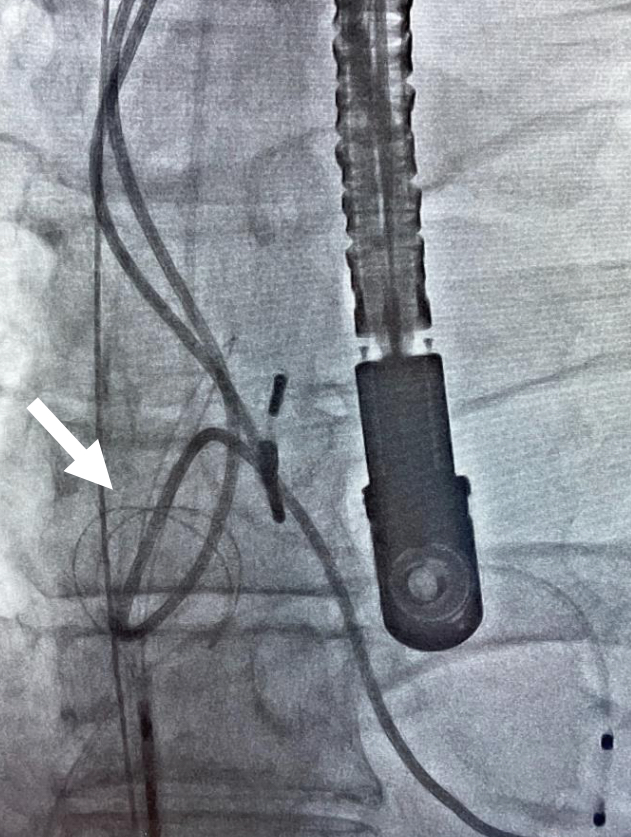
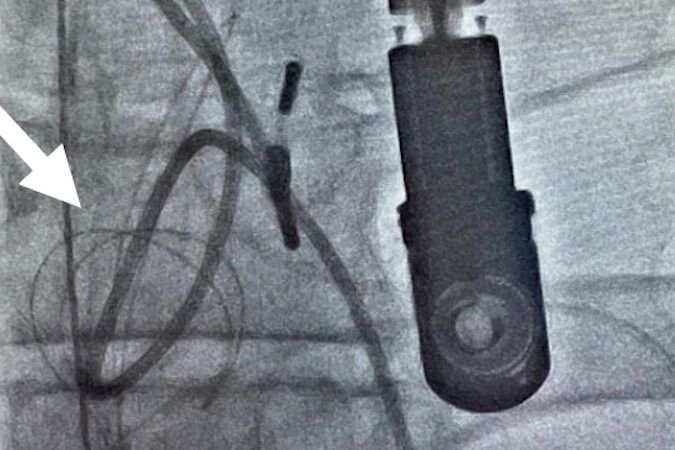
Figure 3: Snare extraction technique from inferior (femoral vein) access (top). Rotational extraction sheath (Cook Evolution) from superior access, over the lead in situ (bottom).
The earliest recorded description of pacing was in 1882 and its now over 60 years since the first patients received an implantable cardiac pacemaker. Many lives have been saved and quality of life improved by these early devices and the many subsequent developments implemented over the following decades. Important developments include the implantable cardioverter defibrillator (ICD) in 1980 and then more recently a treatment for heart failure with cardiac resynchronisation therapy (CRT), first described in 1984 followed by the development in recent years of conduction system pacing (CSP).
Indications for Pacing
Pacemakers are indicated for symptomatic bradycardia and also in cases of atrio-ventricular (AV) block for improving prognosis. In the main cardiac resynchronisation pacemakers are indicated in patients who develop significant intraventricular conduction delay (bundle branch block) or those with left ventricular (LV) impairment who are expected to receive a high degree or percentage of heart beats activated from conventional right ventricular (RV) pacing. Right ventricular pacing is associated with the development of LV impairment (pacing induced cardiomyopathy) and has been shown to increase mortality. Particularly at risk are those with pre-existing LV impairment. Approximately 10-20% of patients with normal LV function will develop LV impairment following significant RV pacing (>40% ventricularly paced beats). ICDs were originally inserted for survivors of cardiac arrest (secondary prevention implantation) but it has become apparent that high risk patients could be identified prior to sudden cardiac death (primary prevention implantation). Current practice is therefore to insert the majority of ICDs for those judged to be at high risk of ventricular arrhythmia as a primary prevention measure. However the prediction of sudden death risk remains challenging although this has been assisted greatly by developments in a number of clinical areas including for example, imaging with cardiac MRI to image myocardial fibrosis and in some specific cardiomyopathies, genetic testing.
Developments in Implantation Techniques
Many developments within surgical specialties have crossed over from operating theatres to the cardiac catheterisation suite. From dissolvable sutures to diathermy and haemostasis techniques, submuscular implantation, regional anaesthesia and nerve blocks. Technological advances in imaging quality and interventional ultrasound techniques have also improved safety in guiding central venous access where standard vein dissection (cut down technique) for pacing lead insertion is not adequate.
Standardised surgical preparation including hair clipping, single dose pre-implantation intravenous antibiotics, normothermia and normoglycaemia have helped minimise infection risk. Further developments have allowed many device procedures to be performed without interrupting anticoagulation reducing the risk of stroke related to withholding anticoagulants. Without the use of diathermy or other haemostasis technique the development of a post procedure haematoma carries a significant increased risk of infection.
Remote Follow Up
Current pacemakers and defibrillators are capable of largely functioning autonomously. Automatic threshold testing reducing battery depletion, detection of arrythmia such as atrial fibrillation and longevity monitoring are just three of the advantages of remote monitoring. Particularly during the COVID era the ability to monitor patients safely remotely has been invaluable. Over the coming this will likely continue to expand and lead to less frequent attendance for patients in person within traditional pacing clinics. Early devices required a specific ‘transmitter’ to be present near the patient, usually left at home to send data to the device company. Newer devices connect to a patients mobile phone through an app from the device company allowing almost continuous connection to the remote monitoring data server. Integration of other biometric data from the mobile phone and the patient alongside the device data will likely provide a more detailed assessment of patients status especially in heart failure and other chronic cardiac conditions.
Magnetic Resonance Imaging in Patients with Devices
MRI conditional devices have become an industry standard across almost all devices from basic pacemakers to defibrillators. Large studies have shown there is no issue in patients who have defibrillators deactivated during scanning. Patients however still require attendance for a pacing check usually immediately preceding and following their MRI to ensure the device is functioning appropriately and safely.
Developments in Bradycardia Pacing
The demand for bradycardia pacing in an ageing population is growing rapidly. An important development has been the recognition of the deleterious effects of a high burden of ventricular pacing. Focus on reducing the risks of these patients developing heart failure from a pacing induced cardiomyopathy has been on software algorithms and more recently techniques to utilise pacing of the patients own conduction tissue. This technique is known as conduction system pacing (CSP) and includes His bundle pacing to target the atrioventricular node and more recently left bundle branch pacing from a lead inserted across the interventricular septum.
Further developments have included leadless pacemakers implanted into the right ventricle. Initially these have been single chamber, ventricular pacing and sensing only devices but more recently an atrial sensing device utilising motion detection of the device corresponding with atrial contraction has become available. Undergoing further clinical evaluation are leadless pacemakers specially for atrial pacing only and devices that are integrated to provide both atrial and ventricular pacing using Bluetooth communication between the devices.
Developments in Internal Defibrillators
Over a number of years battery developments have resulted in both smaller device sizes and also in longer device longevity. This will reduce the frequency of generator changes and the potential complications of such procedures such as infection.
The majority of patients receiving an internal defibrillator have a diagnosis of ischaemic cardiomyopathy or dilated (non ischaemic) cardiomyopathy. Most patients have a primary prevention indication, mainly determined by left ventricular ejection fraction in most conditions. Secondary prevention patients may have had a sustained arrythmia or resuscitated from a cardiac arrest and are also indicated to receive a device in most cases.
Modern imaging techniques such as cardiac MRI (CMR) to assess ventricular function and fibrosis within the mid ventricular wall is also a risk factor for sudden cardiac death in some conditions.
In specific patients the inducibility of sustained ventricular arrhythmia by electrophysiology testing (ventricular pacing) may be used to help guide a decision of defibrillator insertion for a specific patient.
There is a choice of subcutaneous or conventional transvenous internal defibrillators. Most patients receive transvenous systems which can provide ATP (anti tachycardia pacing) to overdrive and terminate a ventricular arrythmia thereby not requiring a device shock or defibrillation. Subcutaneous defibrillators using a lead underneath the skin and not within the vascular system have been shown to be effective and safe, they are able to discriminate between normal rhythms and ventricular tachycardia. Without a transvenous lead within the heart the utilise a surface electrocardiogram (ECG) created between the lead and the defibrillator device instead to detect abnormal rhythms requiring treatment.
Cardiac Resynchronisation Therapy (CRT)
Biventricular pacing to provide cardiac resynchronisation therapy was first described over 25 years ago. CRT has been extensively proven to be one of the most clinically and cost effective cardiovascular therapies in terms of reductions in mortality and heart failure (HF) hospitalisation. Critical to decision making for a patients suitability for this therapy is the presence of electrical conduction delay (bundle branch block) across the heart assessed by the surface ECG QRS duration and pattern, alongside significant LV systolic impairment, ejection fraction <35% in patients receiving optimal heart failure therapy.
However CRT should also be considered for an additional group of patients who are predicted to receive a high degree of right ventricular pacing and who have left ventricular impairment, which is likely to worsen with RV apical pacing. Therefore for a patient requiring a pacemaker in the setting of complete or high grade AV block with reduced LV function should be considered for a CRT device to prevent a deterioration in ventricular function from pacing alone.
Advances is lead implantation techniques and hardware advances including delivery systems and modern quadripolar (vs. bipolar) pacing leads has permitted very high implant rates and avoiding complications such as diaphragm pacing from inadvertent phrenic nerve capture. Improved venous access techniques using intraprocedural ultrasound, venoplasty and balloon dilatation of peripheral and central veins including the superior vena cava have increased procedural success rates and reduced indications for epicardial pacing leads usually applied via an invasive thoracotomy.
Ensuring >92% of heart beats are from CRT is vital, patients with frequent ventricular ectopics or atrial fibrillation require medication alteration, ectopic focus ablation or AV nodal ablation in some to increase the percentage of CRT ventricular pacing therapy, proven to improve outcomes including reducing mortality.
Software innovations to optimise the fusion of intrinsic cardiac conduction, through the AV node and native cardiac conduction tissue and pacing, largely from the left ventricular lead appears to be beneficial. Investigations are ongoing into whether mutlitipoint pacing from a quadripolar left ventricular pacing lead provides benefits that overcome the inevitable battery drain of pacing from more than one site.
Conduction System Pacing (CSP) – His bundle pacing (HBP) and Left bundle branch pacing (LBBp)
Pacing the conduction tissue of the heart directly is an exciting development within cardiology. CSP has the potential to supersede conventional right ventricular pacing. The benefits are in replicating the natural electrical conduction of the heart with a physiological narrow QRS morphology similar to intrinsic conduction. One major benefit of CSP has been its ability to reverse left bundle branch block, potentially replacing conventional CRT in the future once further data is available from upcoming trials. Thus far small trials have shown significant benefits in terms of reduction of heart failure, atrial fibrillation and mortality in those receiving >40% RV pacing. The exact physiological mechanism whereby pacing near the conduction system can reverse left bundle branch block is controversial and much debated
There has been concern that pacing leads inserted into the fibrous His bundle region at the AV node have higher thresholds and therefore reduced battery longevity. A new development of pacing the left bundle branch of the His Purkinjie system, transeptally appears to overcome these issues. So far the technique has been shown to be reproducible, teachable and lower thresholds on these very stable leads in the medium term. Studies in patients undergoing LBBp lead insertion in the setting of heart failure also suggests a response in ejection fraction superior to conventional CRT. This technique of implantation of a pacing lead within the ventricular septum may be combined with conventional left ventricular lead pacing via the coronary sinus (LOT-CRT). Potentially overcoming some of the limitations in resynchronisation of patients with intraventricular conduction delay (within the ventricular conducntion tissue) with conventional techniques. Our own research is assessing this technique against conventional CRT devices, in particular its safety and the learning curve for lead implantation against conventional biventricular pacing.
For some patients often due to the presence of previous leads and potentially occlusion of the superior vena cava access for leads to achieve intracardiac pacing is not possible. Although procedures to cross and recanalize previously occluded central veins is possible for some patients the risks may be significant. Therefore procedures to implant pacing devices either epicardially (thoracoscopically) or endocardially from arterial access retrogradely across the aortic have are possible. One such system uses ‘induction pacing’ with a transmitter inserted initially very similar to a standard pacemaker generator subcutaneously. Followed by the insertion of a pacing ‘seed’ within the left ventricular cavity to pace the myocardiu,. Thereby avoiding the necessity of venous access to pace the left ventricle and achieve cardiac resynchronisation by biventricular pacing if this pacing ‘seed’ is implanted in the left ventricular free wall.
Lead Extraction and Access Management
Many patients require a number of pacing procedures throughout their lifetime. Both through the failure of the leads inserted at their initial implant procedure but also over time if upgrading to more complex systems such as resynchronisation is required. In some cases venous access may also be blocked or narrowed necessitating the use of techniques such as venoplasty to dilate narrowed veins to permit venous access. This may also necessitate the removal of a non functional or previously abandoned lead(s) by extraction of the lead but using this ‘channel’ to pass a wire simultaneously to maintain access for a new lead to be inserted. However if additional leads are added without removing an old lead (extraction) the procedural risks of extraction are avoided but the old leads can increase the risk of future complications:
- Higher risk of future device infection
- More complex future lead extraction (removal)
- Higher risk of occlusion (thrombus or venous stricture of the vein or damage to heart structures
- May complicate or prevent the ability to perform an MRI scan
Extraction of failed leads that may have fractured or require removal due to infection is also an increasing requirement for any device service. Techniques and equipment have evolved significantly to improve both the success of extraction of leads in their entirety as well as the safety of the procedure. Although this complex procedure still requires specific training and competency there is also a requirement for a cardiac surgical back up team in case of emergency. Leads that have been in situ for many years (> 10 years), and in particular specific leads such as dual coil defibrillators leads with a high voltage coil within the superior vena cava or specific lead models known to fragment resulting in significant scar tissue in growth require careful pre procedural planning and consideration of ‘hybrid’ techniques. Hybrid extraction includes not only removing the lead(s) required from the site of venous entry of the lead(s) but the assistance of snaring the lead as well to provide counter traction either from a superior jugular vein of inferiorly from the inferior vena cava. Despite these developments lead extraction remains challenging and with significant risks and is therefore performed in specific centres like ours with appropriate expertise. However in published studies high volume operators show clinical success rates of around 98% for successful lead removal. The risk of internal bleeding, cardiac tamponade or haemothorax among other risks is 0.5%. With an ageing population, improvements in heart failure and increasing implantation indications it is inevitable that we will see a rapid increase in all device related procedures over coming years.